The Biden administration’s 100-day review of critical supply chains has yielded detailed policy recommendations across four major sectors including technology-focused products including large-capacity batteries, semiconductors and the key minerals they depend on as discussed in Panjiva’s June 17 report. The fourth sector of pharmaceuticals and active pharmaceutical ingredients (APIs) has come into focus largely as a result of the pandemic and the ongoing challenges faced in supplying both U.S. citizens as well as global demand with vaccines.
The report includes six broad recommendations linked to pharmaceuticals and APIs assembled by the Department of Health and Human Services (DHHS). Most are aimed at the long-term development of production of pharmaceuticals and APIs in the U.S. including investment in R&D, improved supply chain transparency and international coalition building.
There are two elements that may result in near-term spending in the sector. One is to provide direct financial incentives to boost domestic production, with a near-term step to be to identify the top 50 – 100 drugs that “are most critical to have available at all times for U.S. patients because of their clinical need and lack of therapeutic redundancy” as identified during the pandemic.
The second includes exploring the “creation / expansion of a virtual strategic stockpile of APIs … and other critical materials” as well as potentially a stockpile of vaccine doses against future pandemics.
The administration will also have to address the thorny issue of API exports with purchasing by a strategic stockpile potentially crowding out buyers from other countries that have smaller financial means. Thus far the Biden administration has not pursued a bar on exports, as discussed in a recent paper by Panjiva Research and the Peterson Institute for International Economics. That may change given DHHS recommends a reconstruction of mechanisms used in the Defense Procurement Act.
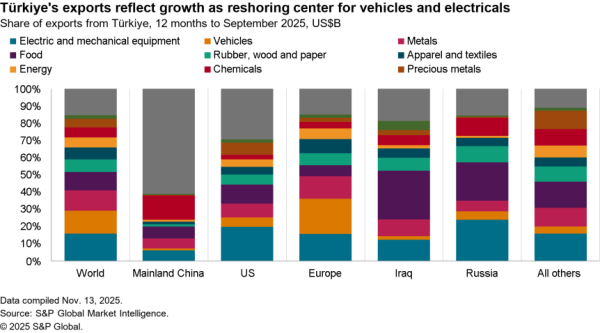
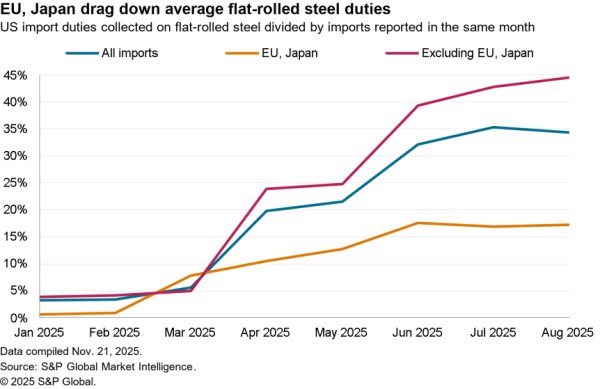
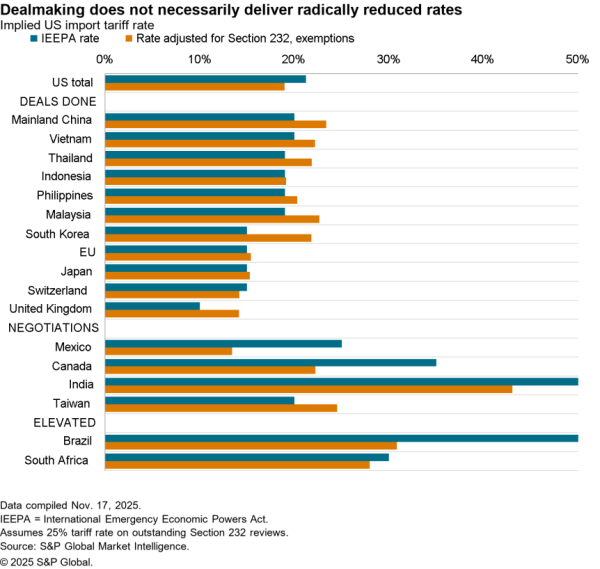
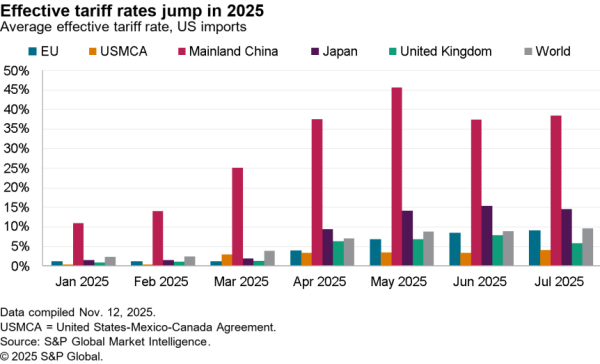
There is nonetheless some evidence of a small slowdown recently with exports of APIs in April dipping 5.1% compared with the level of the prior three months. At $506 million they were also just one-third the level of imports. Nonetheless, there was a 59.7% surge in shipments to India and a 40.1% rise in exports to China which crowded out shipments to Europe and Canada / Mexico which fell by 9.4% and 6.2% respectively in April.

Source: Panjiva
The passing of the pandemic does not mean there is no urgency in implementing the administration’s plans, however. One issue for purchasing has come from an increased reliance on China for APIs during the past year. Panjiva’s data shows that the share of imports of a wide range of APIs, not just for vaccines, coming from China increased to 18.4% in the three months to April 30 compared to 14.1% the same period a year earlier.
That likely displaced shipments from the EU which fell to 49.9% of the total from 55.5% over the same period. Total API imports have increased by 5.6% year over year in the past three months with those from China surging 38.0% and those from Europe dipping 5.1%. There’s also been an increase in shipments from Switzerland, which climbed 57.6% year over year while imports from the U.K. dropped by 53.4%.

Source: Panjiva